Burdensome therapeutic protocols can physically and psychologically impact patients and caregivers1,2
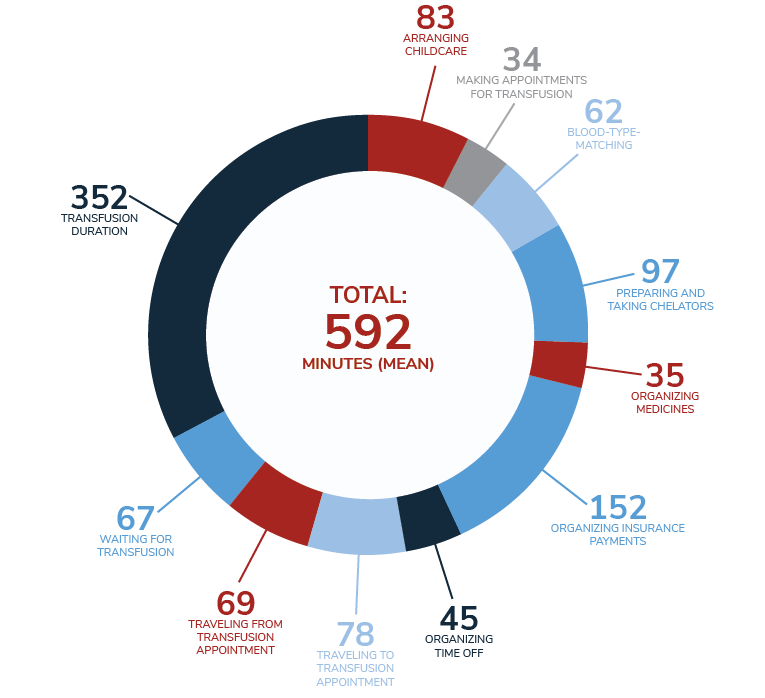
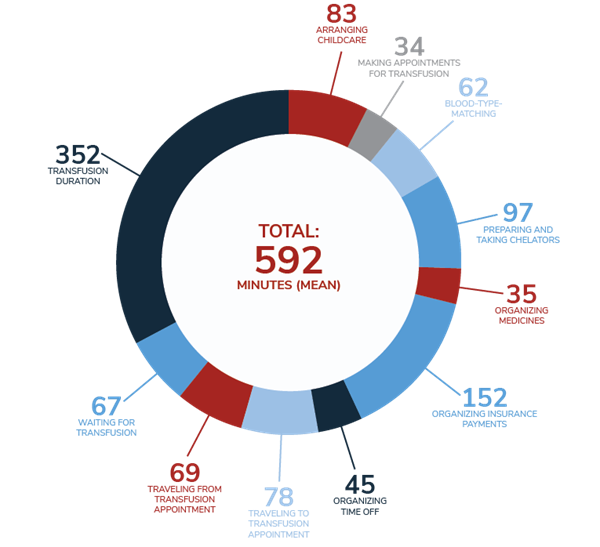
The process of receiving a transfusion can take up an entire day when travel, blood tests and results, transport to the transfusion center, and the transfusion itself are considered.3 In the 2019 MyThalLog study,* patients and caregivers reported the mean transfusion frequency as every 3.2 weeks, with the mean time spent on TDT management being 9 hours and 52 minutes on transfusion days and 91 minutes on non-transfusion days (11 hours per week=528 hours per year).4
*A bluebird bio–sponsored prospective, observational, real-world study of adults with TDT and caregivers of adolescents with TDT (N=85) living in the UK, USA, Italy, and Germany. Over 90 days, participants used a smartphone app to respond to daily surveys about their or their dependent’s TDT, including background and disease-management surveys, Brief Fatigue Inventory (BFI), the Transfusion-dependent Quality of Life (TranQoL), and the Brief Pain Inventory Short Form.4
Mean daily time spent (in minutes) on TDT management activities on transfusion days4
Patient and caregiver self-reported data† show burden of TDT is high4
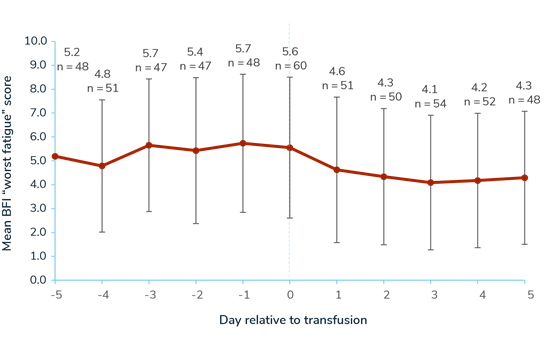
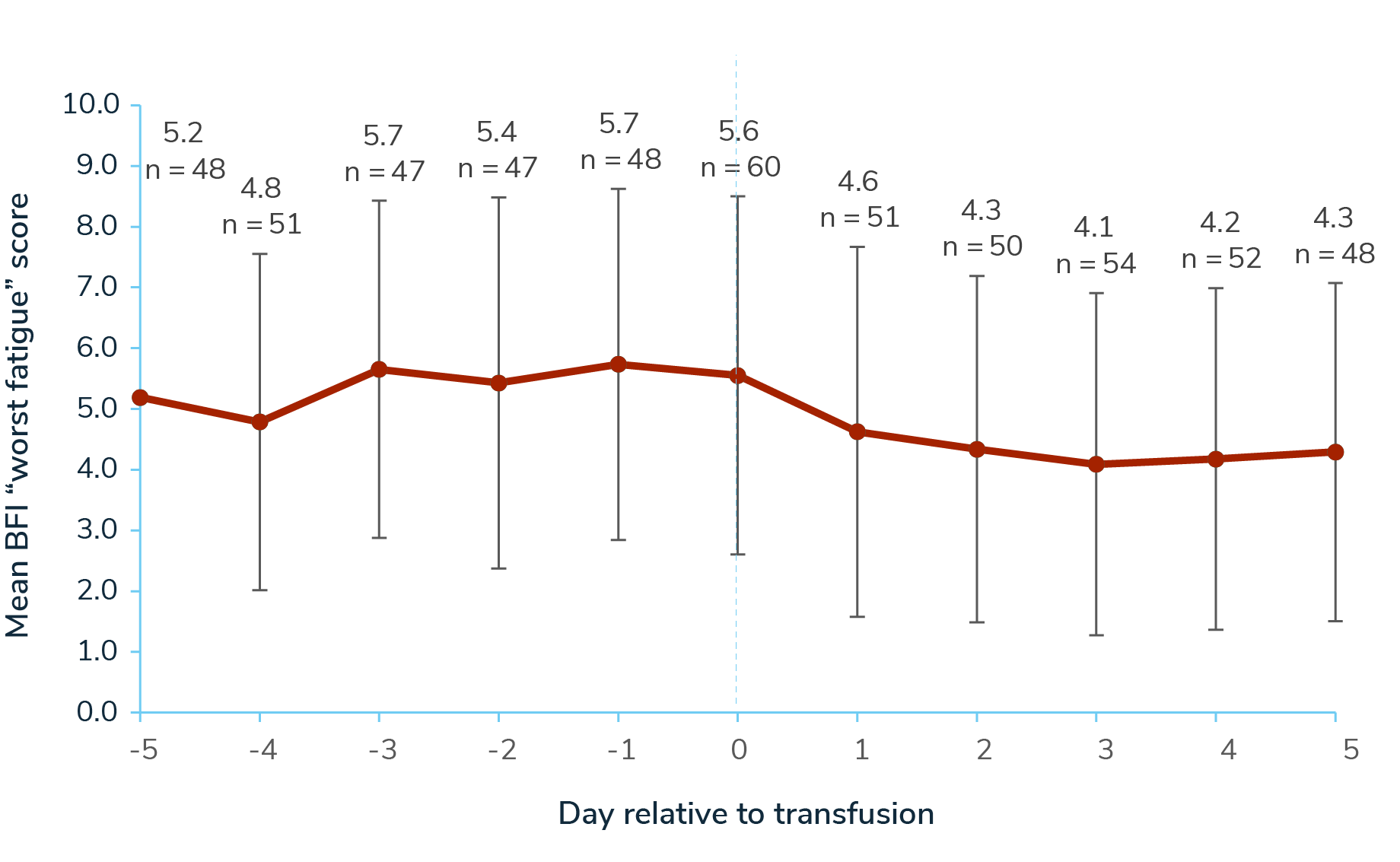
Across the 5-day periods pre- and post-transfusion, 63 participants responded to a Brief Fatigue Inventory (BFI) “worst fatigue” questionnaire. Mean “worst fatigue” score was 5.05 (SD 2.83) in the 5 days pre-transfusion, 5.55 (SD 2.95) on transfusion days, and 4.29 (SD 2.81) in the 5 days post-transfusion (0–10 scale; 10=worst symptoms).4
Mean Brief Fatigue Inventory “Worst Fatigue” Score Relative to Transfusion Day (0-10 scale; 10=worst fatigue)
 SWIPE OR ROTATE
SWIPE OR ROTATE
Separately, 73 participants responded to a Transfusion-dependent Quality of Life questionnaire (TranQoL), which assesses HRQoL across the following domains: emotional health, family functioning, career/school functioning, and physical health. The mean score was 51 (0-100 scale, 100=best quality of life).4
Mean TranQol Domain and Overall Scores (0-100 scale; 100=best HRQoL)4
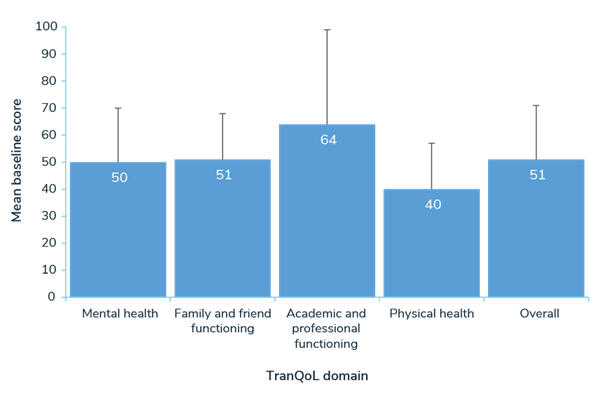
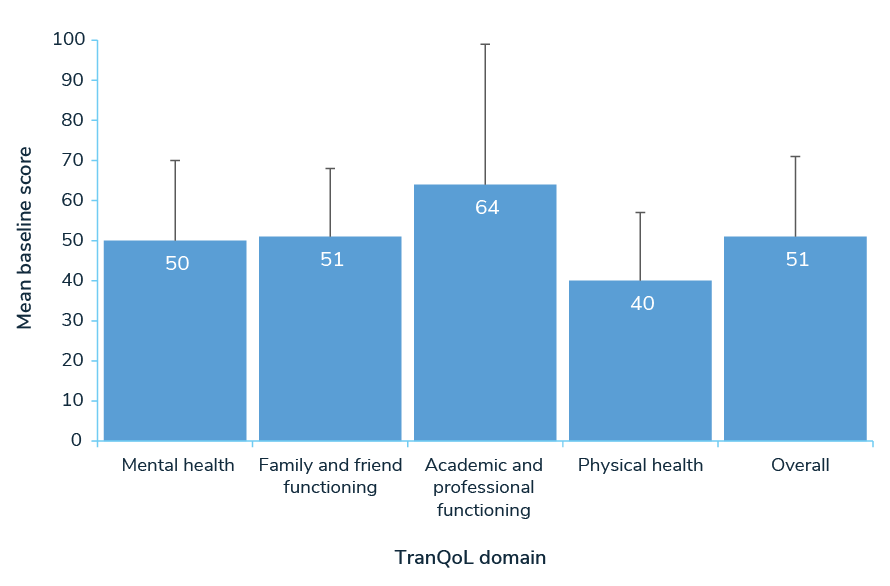
TranQoL = Transfusion-dependent Quality of Life.
Adapted from Paramore C, Levine L, Bagshaw E, et al. Patient- and caregiver-reported burden of transfusion-dependent β-thalassemia measured using a digital application. Patient. Published online October 30, 2020. doi:10.1007/s40271-020-00473-0
*A bluebird bio–sponsored prospective, observational, real-world study of adults with TDT and caregivers of adolescents with TDT (N=85) living in the UK, USA, Italy, and Germany. Over 90 days, participants used a smartphone app to respond to daily surveys about their or their dependent’s TDT, including background and disease-management surveys, Brief Fatigue Inventory (BFI), the Transfusion-dependent Quality of Life (TranQoL), and the Brief Pain Inventory Short Form.4
†Several limitations of our study design must be considered when interpreting the results. First, given the app-based approach, the population was restricted to confident smartphone users. This may have excluded some potential participants who were older, lacked access to a smartphone, or had difficulties with vision or dexterity. The remote, app-based approach also increased the challenges of verifying participant eligibility and ensuring the accuracy of self-reported data. Our study was also limited by the fact that each survey was analyzed independently, with participants included in the analysis if they responded to that particular survey, rather than if they responded to all surveys or, at a minimum, the background demographic survey. Another limitation was the short data-collection period, which meant that our results may not be representative of the long-term burden of TDT. Another factor limiting the quality of our study results was the use of a novel disease-management survey. One final area of the study design that may have had undesired effects was the financial reward system used to encourage participant engagement.4
Although the app-based approach taken in this study introduced several potential limitations, it did provide some advantages over in-person methods, which should be noted. The ease with which participants could learn about the study, enroll, and contribute data promoted a large, diverse, and complete dataset, while digital data management and analysis reduced administrative burden and opportunities for human error.4
Adapted from Paramore C, Levine L, Bagshaw E, et al. Patient- and caregiver-reported burden of transfusion-dependent β-thalassemia measured using a digital application. Patient. Published online October 30, 2020. doi:10.1007/s40271-020-00473-0
*A bluebird bio–sponsored prospective, observational, real-world study of adults with TDT and caregivers of adolescents with TDT (N=85) living in the UK, USA, Italy, and Germany. Over 90 days, participants used a smartphone app to respond to daily surveys about their or their dependent’s TDT, including background and disease-management surveys, Brief Fatigue Inventory (BFI), the Transfusion-dependent Quality of Life (TranQoL), and the Brief Pain Inventory Short Form.4
†Several limitations of our study design must be considered when interpreting the results. First, given the app-based approach, the population was restricted to confident smartphone users. This may have excluded some potential participants who were older, lacked access to a smartphone, or had difficulties with vision or dexterity. The remote, app-based approach also increased the challenges of verifying participant eligibility and ensuring the accuracy of self-reported data. Our study was also limited by the fact that each survey was analyzed independently, with participants included in the analysis if they responded to that particular survey, rather than if they responded to all surveys or, at a minimum, the background demographic survey. Another limitation was the short data-collection period, which meant that our results may not be representative of the long-term burden of TDT. Another factor limiting the quality of our study results was the use of a novel disease-management survey. One final area of the study design that may have had undesired effects was the financial reward system used to encourage participant engagement.4
Although the app-based approach taken in this study introduced several potential limitations, it did provide some advantages over in-person methods, which should be noted. The ease with which participants could learn about the study, enroll, and contribute data promoted a large, diverse, and complete dataset, while digital data management and analysis reduced administrative burden and opportunities for human error.4
When compared with other chronic diseases, self-reported BFI levels (mean 5.0 at enrollment of 2019 study) were similar to Crohn’s disease (4.2), ulcerative colitis (4.1), and rheumatoid arthritis (7.3)4
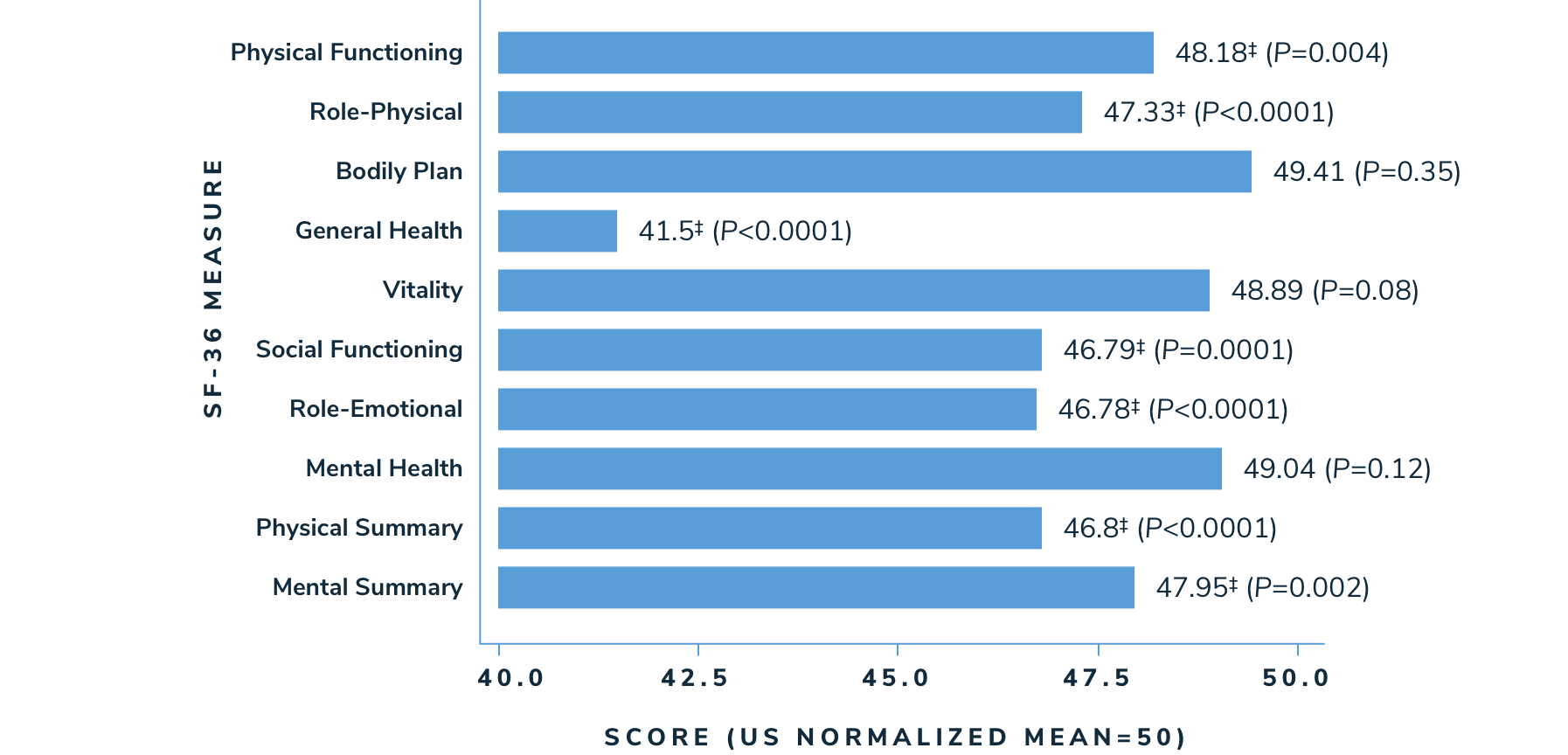
Patients with thalassemia reported having a lower HRQoL in multiple SF-36 measures than the general population1
According to a longitudinal cohort study looking at health-related quality of life (HRQoL) in 264 thalassemia patients >14 years of age in North America (n=229) and the United Kingdom (n=35)1:
- Adolescent and adult patients in the Thalassemia Longitudinal Cohort (TLC) had significantly worse HRQoL on 5 of the 8 subscales (physical functioning, role-physical, general health, social functioning, and role-emotional; P<0.05), and on both summary scales (physical component summary and mental component summary)
- Lower HRQoL was also reported in women, older patients, and those who had more disease complications and chelation-related side effects
Health-Related Quality of Life Scores (SF-36‡) in the Thalassemia Longitudinal Cohort vs US Norms1
 SWIPE OR ROTATE
SWIPE OR ROTATE


Adapted from Sobota A, Yamashita R, Xu Y, et al. Quality of Life in Thalassemia: A Comparison of SF-36 Results from the Thalassemia Longitudinal Cohort to Reported Literature and the US Norms. Am J Hematol. 2011;86(1):92-95.
‡These health domains are evaluated as part of the Medical Outcomes Study Short Form 36-Item (SF-36). They are defined as follows: physical functioning covers limitations in daily life due to health problems. The role-physical scale measures role limitations due to physical health problems. The bodily pain scale assesses the frequency of pain and interference of pain with usual roles. The general health scale measures individual perceptions of general health. The vitality scale assesses energy levels and fatigue. The social functioning scale measures the extent to which ill health interferes with social activities. The role-emotional scale assesses role limitations due to emotional problems.5 From: Busija L, Pausenberger E, Haines T, et al. Adult Measures of General Health and Health-Related Quality of Life Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res. 2011;63(11):S383-412.
§One sample T-test shows significant difference between population and US norm (P<0.05).
‡These health domains are evaluated as part of the Medical Outcomes Study Short Form 36-Item (SF-36). They are defined as follows: physical functioning covers limitations in daily life due to health problems. The role-physical scale measures role limitations due to physical health problems. The bodily pain scale assesses the frequency of pain and interference of pain with usual roles. The general health scale measures individual perceptions of general health. The vitality scale assesses energy levels and fatigue. The social functioning scale measures the extent to which ill health interferes with social activities. The role-emotional scale assesses role limitations due to emotional problems.5 From: Busija L, Pausenberger E, Haines T, et al. Adult Measures of General Health and Health-Related Quality of Life Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res. 2011;63(11):S383-412.
§One sample T-test shows significant difference between population and US norm (P<0.05).
Caregivers of children with beta-thalassemia also reported to have decreased HRQoL, and to have experienced impaired physical and psychological health.2
Creating an ongoing conversation in which caregivers can communicate openly and honestly may lead to insights about their concerns and can help you create and adjust a medical treatment plan together.
Consider asking your patients with TDT:
“How would you describe a good day and a bad day related to TDT?”
“How would you describe a good day and a bad day related to TDT?”

Setting goals: A guide for patients
Help your patients develop goals and have deeper discussions about their care
Actor portrayal. Not a real patient.
Take the Beta-Thalassemia
Challenge
In a longitudinal cohort of 264 thalassemia patients, beta-thalassemia patients reported having lower health‑related quality of life (HRQoL) than the normal population in which health domains?